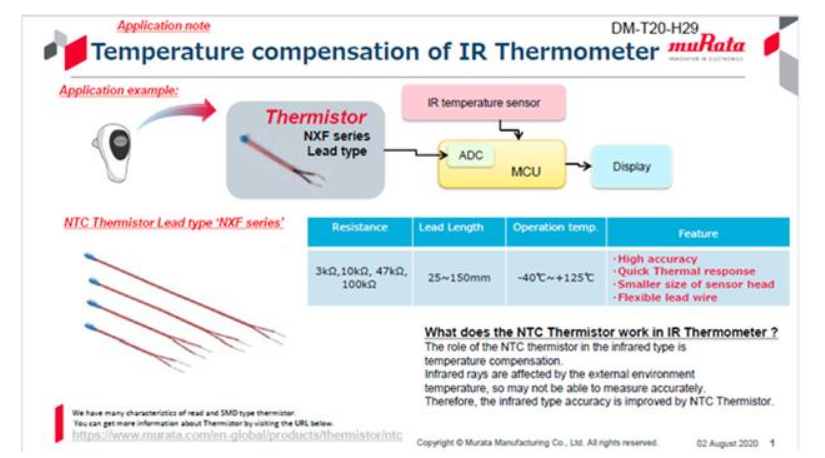
Overview
NTC thermistors can be used to sense temperature, and RFID tags can be used to track and manage medical supplies. This article introduces NTC thermistors and RFID products from Murata and summarizes their characteristics for reference in product development.
NTC Thermistors as Temperature Sensors
NTC (Negative Temperature Coefficient) thermistors are resistive devices whose resistance decreases as temperature rises. They are made from sintered non-oxide ceramics composed of manganese, nickel, cobalt, and other elements, with electrodes formed on the ceramic body. Common package types include leaded styles and SMD chip types. Resistance changes with temperature and can range roughly from 1% to 5% per °C, making NTC thermistors a common choice for electronic temperature sensing.
Murata's NTC thermistors are offered with high accuracy and fast thermal response. The product range includes various package shapes (SMD chips and leaded types) and targets applications such as temperature monitoring, overheat detection, circuit protection, current control, and heating. The NXF series is a flexible leaded type suited for temperature sensing. NXF parts are compact and fast-responding, with flexible lead lengths available from 25 to 150 mm. Murata's ceramic processing allows these thermistors to meet a range of application requirements.
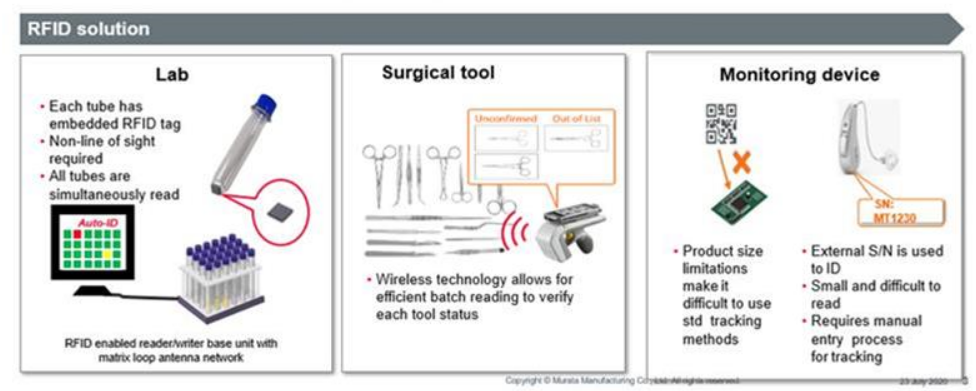
RFID Tags for Medical Device Management
During surgical procedures, many different instruments are used, and managing those instruments is a significant operational challenge. Regulations such as the U.S. Unique Device Identifier (UDI) rule require device identification to support safe use and traceability; similar regulations have been adopted or planned in other regions. Given the wide variety of medical instruments and their complex shapes, manual recordkeeping is inefficient and error prone. Traditional approaches such as barcodes, laser marking, or QR codes have clear limitations: barcodes are difficult to read on instruments, laser marking may increase the risk of corrosion or contamination, and QR-code scanning is time consuming. RFID-based solutions implemented on reliable medical PCB platforms offer clear advantages for surgical instrument tracking.
Fast, bulk RFID reads enable efficient instrument set assembly and inspection. Use frequency and cumulative operating time can be recorded and viewed on PCs or other systems, helping to reduce excess inventory and optimize preoperative instrument preparation.
Murata's LXTBKZMCMG-010 is a UHF RFID tag designed for surgical instruments and medical devices on metal surfaces. It is a compact module that can be attached to metal objects and supports global use with high reliability. The LXTBKZMCMG-010 uses the UHF band (865-928 MHz), complies with ISO 18000-63 / EPC Global Gen2 (v2), and measures 6.0 x 2.0 x 2.3 mm. It uses Impinj Monza R6P and achieves a maximum read range on metal up to 150 cm (4 W EIRP). The module is RoHS compliant.
Murata provides RFID tags suitable for metal applications and reports that some tags withstand high-temperature sterilization processes. RFID tags can be read with handheld readers or fixed readers and antennas to capture all instruments on a table in real time without manual scanning. Typical read ranges vary by environment and setup and can span approximately 10 cm to 1 m depending on conditions.
Traceability for Small Products
Small, customizable products such as hearing aids require per-item records for manufacturing data and personalized settings. Due to miniaturization, conventional visible marking methods like QR codes become impractical. Hearing aids frequently contact skin, and external labels can become soiled, hindering identification. RFID tags support centralized management from manufacturing through after-sales service; tags embedded inside a product can still be read externally, and stored data is rewritable and resistant to surface contamination.
Murata's LXMSJZNCMF-198 and LXMS21NCNH-147 are UHF RFID modules designed for monitoring small products such as hearing aids, tracking devices, medical kits, and labware. These modules are compact and robust and can be attached to or embedded in metal or non-metal objects using adhesives, double-sided tape, or overmolding. LXMSJZNCMF-198 operates in the UHF band (865-928 MHz), complies with ISO 18000-63 / EPC Global Gen2v2, measures 1.2 x 1.2 x 0.55 mm, uses Impinj Monza R6, and has a typical read range around 10 mm. LXMS21NCNH-147 has similar characteristics but complies with ISO 18000-63 / EPC Global Gen2 (v1.2.0), measures 2.0 x 1.25 x 0.55 mm, and uses NXP G2iM.
These RFID modules can be mounted on substrates and overmolded to enable product-level history tracking from manufacturing onward. Support is available for antenna design and tag integration to help improve production workflow and after-sales processes.
RFID for Medication Safety
Medication errors remain a concern in clinical settings. RFID can be applied to pharmaceutical control, for example by tagging ampoules to prevent wrong drug administration and to automatically record dosages and usage times. Tags embedded in the drug container rather than just the outer packaging help preserve identification when the item is removed from its box. An individual ampoule's RFID memory can store device configuration and prescription history.
Murata's RFID tags have compact, robust designs suitable for embedding while preserving current product form factors. RFID memory areas (user memory and EPC memory) are rewritable, and password locking can prevent unauthorized modification. Each IC contains a unique, non-rewritable serial number for product verification. Tags can be attached with adhesive or embedded by overmolding, and non-contact reading avoids wear. Reader and tag communication can remain reliable even if contaminated by chemicals or other substances.
Conclusion
NTC thermistors are suitable for electronic thermometers and other temperature sensing applications due to their sensitivity and stable response. RFID technology supports medical safety and traceability by improving data accuracy, reducing manual errors, and enabling lifetime tracking and return-material traceability. These component families provide engineers with options for integrating temperature sensing and identification capabilities into medical devices and consumables.