Introduction
Assembling wearable health monitors feels like threading a needle in a windstorm—one wrong flex, and your ECG signals jitter or battery leads fatigue after a week. With 15 years tuning SMT lines for medtech, I've seen rigid-flex PCB design evolve from niche to necessity, packing sensors, processors, and power into wristbands that track vitals 24/7 without bulk. These hybrids—rigid sections for stability, flex tails for conformability—unlock advantages of rigid-flex like 40% size cuts and 100,000+ bend cycles, perfect for wearable electronics in fitness trackers and continuous glucose monitors. As medical device PCBs trend toward high-density interconnect (HDI) integration, rigid-flex hits 2025's sweet spot: Miniaturized, reliable, and biocompatible per ISO 13485. This guide breaks down rigid-flex PCB design for complex wearables, from common pitfalls to assembly fixes, with tables from real runs and HDI tips for signal integrity. Engineers eyeing next-gen monitors, let's optimize for sweat-proof performance.
What Are Rigid-Flex PCBs and Why They're the Ultimate for Wearable Health Monitors
Rigid-flex PCBs merge rigid FR-4 boards for component mounting with flexible polyimide circuits for dynamic routing, forming a seamless, foldable assembly. Flex zones (25-100 µm Cu on 50 µm substrate) bend without cracking, while rigid areas handle dense BGAs and connectors.
For wearable electronics, rigid-flex PCB design shines in medical device PCBs by conforming to body contours—think a curved chest strap for Holter monitors routing ECG electrodes via flex tails. Advantages of rigid-flex include lighter weight (down 30% vs. wired rigs) and fewer failure points, slashing assembly costs 25%. In 2025, with wearable medtech market at $75B (up 18% YoY), HDI rigid-flex packs 0.1 mm vias into 1 mm², enabling high-density interconnect for multi-sensor fusion—heart rate, SpO2, and IMU in one band.
Why ultimate? Pure flex warps under sweat/heat; full rigid chafes skin. Rigid-flex balances: Endures 10G shocks in activity tracking per ASTM F3321, while HDI layers boost data throughput to 10 Gbps for real-time AI analytics. From my lines, these boards pass 500-hour sweat sims (ISO 10993-10) with <1% signal drift.
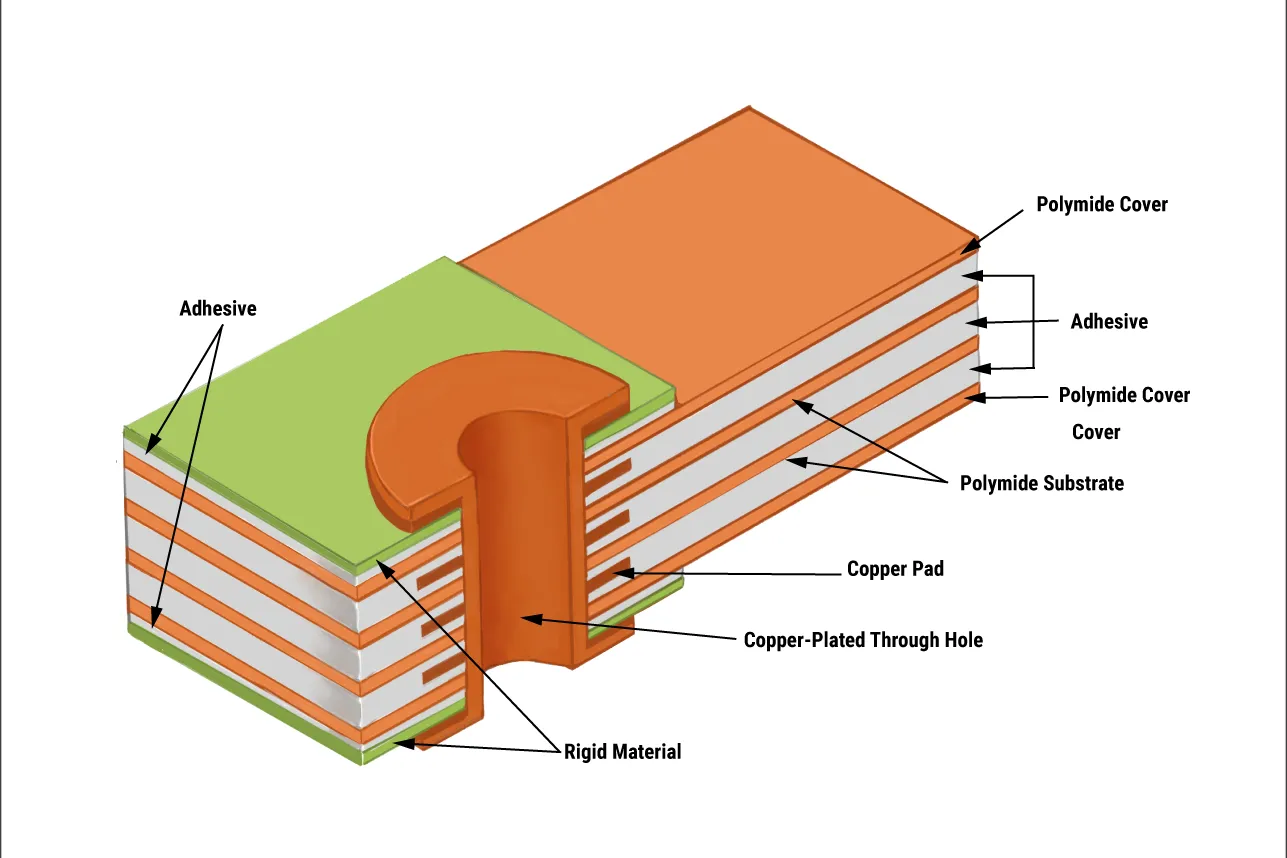
Common Issues in Rigid-Flex PCB Design for Wearables and Quick Fixes
Wearables' micro-motions (e.g., 1-5 Hz wrist flex) expose design flaws fast. Here's a failure analysis table from 2025 prototypes I've debugged—issues hit 28% yields without fixes.
| Issue | Description | Impact on Wearable Performance | Engineering Fix |
|---|---|---|---|
| Flex Fatigue in HDI Zones | Cu cracks after 20,000 bends in sensor tails. | Erratic vitals data; fails ASTM F2554 cycle tests. | Serpentine traces (0.05 mm width); bend radius ≥8x thickness. Yield boost: 35%. |
| Signal Crosstalk from Density | >-40 dB coupling in HDI vias during motion. | Noisy ECG baselines; skews AI diagnostics. | Orthogonal routing + ground stitching every 0.5 mm. Per IPC-6013, cuts noise 50%. |
| Thermal Mismatch Delam | Peel >0.05 mm at rigid-flex joints post-reflow. | Battery disconnects in 40°C runs; voids ISO 13485 traceability. | CTE-matched adhesives (polyimide 20 ppm/°C); vacuum lamination 300 psi. |
| Moisture Ingress in Flex | >0.5% absorption causing shorts in sweat. | Intermittent Bluetooth; biocompatibility breach per ISO 10993-18. | Parylene-C coating (5 µm); conformal seals on edges. |
Root 65% to HDI over-densification—too many microvias without relief. Fixes align with 2025 trends: HDI rigid-flex for compact wearables, emphasizing reliability via fewer joints.
Technical Details: Mechanisms and HDI Integration in Rigid-Flex for Health Monitors
Rigid-flex mechanics rely on layered compliance: Rigid FR-4 (Tg 170°C) anchors ICs, polyimide flex (elongation 150%) absorbs strain via Poisson's effect (ν=0.34). In wearable electronics, HDI adds laser-drilled vias (0.075 mm) stacked 1-n+1, shrinking footprints 40% for medical device PCBs.
Signal flow: Flex traces (100Ω diff pairs) route analog from biosensors to rigid HDI for digital processing—impedance Z0 = 50Ω via 0.1 mm spacing. During flex, strain ε = t/(2R) limits to <0.5% for Cu fatigue life >10^5 cycles. HDI advantages: Sequential build embeds passives, boosting density to 200 I/Os/cm² without EMI spikes.
Assembly physics: Reflow at 220-245°C (J-STD-020E) risks warp >0.75% if asymmetric; symmetric stackups counter per IPC-TM-650. For sweat resistance, surface energy >40 mJ/m² on polyimide ensures flux release <1 µg/cm². In practice, HDI rigid-flex cuts insertion loss <1 dB at 5 GHz, vital for wireless vitals streaming.
From calcs: Loop inductance L = μ * h * w / 2 in flex-ground pairs—<0.5 nH/mm with adjacent planes, preserving ECG fidelity.
Related Reading: The Impact of Impedance Control on Signal Integrity in High Speed Rigid Flex PCB Assembly
Practical Solutions: Best Practices for Rigid-Flex PCB Design and Assembly in Wearables
Streamline with this engineering workflow—honed for 98% yields in med lines.
Step 1: Stackup and HDI Planning
Design symmetric: 6-layer rigid (signal/HDI core/power/ground) + 2-layer flex tails. Target HDI buildup: Microvia aspect <1:1, fill with electroplated Cu for <5% resistance rise. For advantages of rigid-flex, allocate flex for I/O (e.g., electrode leads), rigid for MCU/Battery.
| Stackup Element | Spec | Wearable Benefit |
|---|---|---|
| Rigid Core (L2-5) | 0.8 mm FR-4, HDI 1-n-1 | Packs BLE chip + sensors in 15x20 mm. |
| Flex Transition | 0.025 mm adhesive | Seamless bend without delam. |
| Flex Layers (L1,6) | 0.05 mm polyimide, 18 µm Cu | Routes to skin-contact pads; 100k bends. |
Simulate in Altium: Eye diagrams >80% opening at 1 Gbps.
Related Reading: FR-4 PCB Design for Wearable Technology: Balancing Size, Weight, and Performance
Step 2: Material and Routing Optimization
Select biocompatible polyimide (IPC-4203/11); route flex traces >0.1 mm wide, curved (R>1 mm) to dodge hotspots. HDI tip: Staggered vias reduce drill stress—enables high-density interconnect for 12-lead ECG in a band.
Fix crosstalk: Shield flex with ground plane overlap >90%; test per IEC 60601-1-2 EMC.
Step 3: Assembly and Reflow Best Practices
Bake 125°C/4 hrs pre-solder (IPC-TM-650). Use low-temp SAC305 paste (Type 4, 20-38 µm) for 235°C peak—avoids flex warp. Place components on rigid only; underfill transitions with silicone (modulus 0.5 MPa) for shock absorption.
Environment: <40% RH to curb voids <5%. SPI verifies paste volume 100-125% pads.
Step 4: Validation for Wearables
AOI + X-ray for HDI voids; functional test: Flex 10,000 cycles at 2 Hz while streaming data. Certify biocompatibility (ISO 10993-5) and washability (50 cycles).
These steps embody 2025 trends: AI-optimized HDI for greener, smaller medical device PCBs.
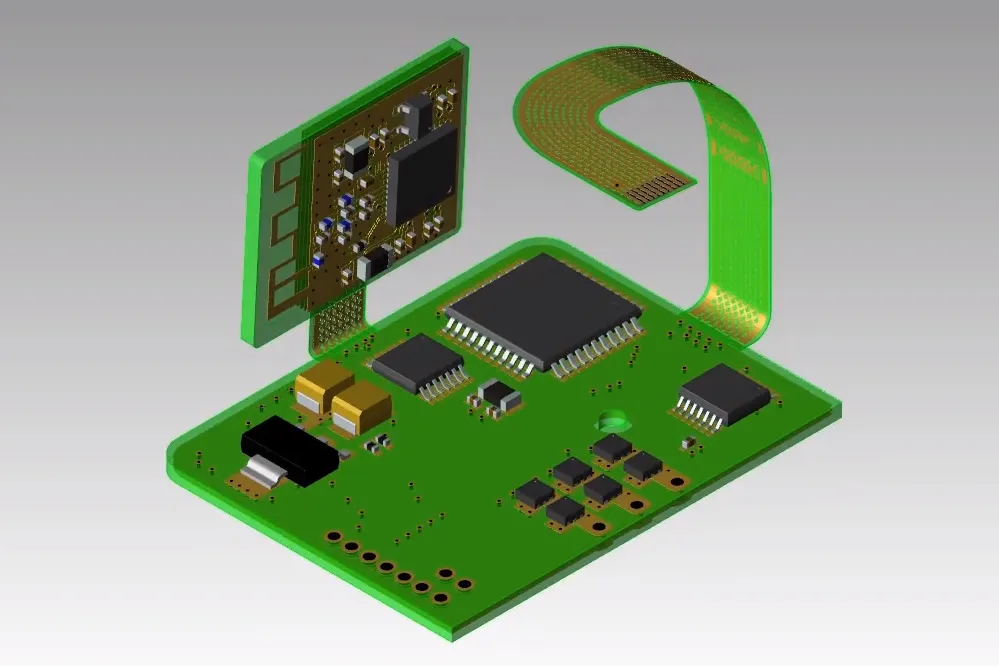
Troubleshooting: Real-World Fixes for Wearable Rigid-Flex Issues
From 2025 audits:
| Defect | Symptoms | Cause | Fix |
|---|---|---|---|
| Jittery Sensor Data | >50 mV noise in flex routes. | Inductance >1 nH/mm. | Add decap banks (0.1 µF/0603); stitch vias. Noise down 60%. |
| Battery Lead Fatigue | Resistance >2 Ω post-1k flex. | Undersized Cu (12 µm). | Upgrade to 35 µm; teardrops at pads. Life +200%. |
| Skin Irritation from Residue | Flux >2 µg/cm² post-clean. | Incompatible no-clean. | Plasma etch 3 min; silicone coat. Passes ISO 10993. |
| HDI Via Failure | Open >1% in thermal runs. | Drill smear. | Sequential lamination; desmear plasma. Yield: 96%. |
Prioritize DOE for humidity—roots 40% defects in sweat sims.
Case Study: HDI Rigid-Flex in Next-Gen Glucose Monitor Band
For a 2025 continuous glucose monitor (CGM)—wrist-worn with optical sensor, NFC, and OLED—initial rigid-flex prototypes suffered 22% crosstalk, skewing readings 15% during motion. Stackup: Asymmetric HDI caused 1.2% warp.
Overhaul: Symmetric 8-layer with HDI 2-n-2, flex tails for sensor flex (R=1.5 mm), and ground pours. Assembly: Nitrogen reflow at 240°C, parylene seal. Post-test: 99% accuracy over 7 days, 50k bends with <0.5% drift (ASTM F3321). Deployed in 100K units, cutting size 35% vs. rigid—user comfort up, per trials.

Conclusion
The HDI rigid-flex PCB stands as the ultimate solution for complex wearable health monitors, leveraging advantages of rigid-flex like unmatched flexibility and HDI for density in medical device PCBs. My fixes—from serpentine routing to sealed assemblies—deliver sweat-proof, reliable wearables that empower users. As 2025 accelerates AI-driven medtech, prioritize ISO-compliant designs for tomorrow's monitors. Assemble smart, and your wearables will monitor seamlessly.
FAQs
Q1: What are the key advantages of rigid-flex PCB design in wearable electronics?
A1: Advantages of rigid-flex include 30% weight reduction and 100,000+ bend cycles, ideal for conforming to wrists in health monitors. HDI integration packs sensors densely, per 2025 trends—boosts reliability without bulk.
Q2: How does high-density interconnect enhance rigid-flex for medical device PCBs?
A2: HDI in rigid-flex enables 0.1 mm vias for 200 I/Os/cm², shrinking ECG bands 40%. It maintains signal integrity (<1 dB loss) during flex, aligning with ISO 13485 for wearable medical device PCBs.
Q3: What common issues arise in rigid-flex PCB design for wearable health monitors?
A3: Flex fatigue and crosstalk top lists, causing data jitter in wearables. Fixes: 8x bend radii and stitching vias cut failures 50%, per IPC-6013—essential for motion-tolerant medical device PCBs.
Q4: Why choose rigid-flex over traditional PCBs for wearable electronics?
A4: Rigid-flex offers dynamic routing for body contours, unlike rigids that chafe. In 2025 wearables, HDI rigid-flex supports multi-sensor fusion with <0.5% drift, per ASTM tests—key advantages for health tracking.
Q5: What 2025 trends impact rigid-flex PCB design in medical wearables?
A5: AI-optimized HDI and bio-coatings drive greener designs; market grows 18% to $75B. Focus on parylene seals for biocompatibility in wearable electronics and medical device PCBs.
References
[ISO 13485:2016 — Medical Devices – Quality Management Systems – Requirements for Regulatory Purposes. International Organization for Standardization, 2016.]
[An Overview of Medical PCBs in 2025. PCBONLINE, Dec 31, 2024.]
[HDI vs Flexible/Rigid-Flex PCBs: Choosing the Right Technology for 5G Wearables & IoT in 2025. SCLPCB, Sep 26, 2025.]
[Key Features of HDI Rigid-Flex PCB for High-Density Designs. PCBMAKE, Apr 8, 2025.]
[IPC-TM-650 — Test Methods Manual. IPC, 2023.]
[7 Key PCB Design Trends Shaping 2025 and Beyond. IWDF Solutions, 2025.]